Filters
Host (768597)
Bovine (1090)Canine (20)Cat (408)Chicken (1642)Cod (2)Cow (333)Crab (15)Dog (524)Dolphin (2)Duck (13)E Coli (239129)Equine (7)Feline (1864)Ferret (306)Fish (125)Frog (55)Goat (36847)Guinea Pig (752)Hamster (1376)Horse (903)Insect (2053)Mammalian (512)Mice (6)Monkey (601)Mouse (96266)Pig (197)Porcine (70)Rabbit (358709)Rat (11723)Ray (55)Salamander (4)Salmon (15)Shark (3)Sheep (4247)Snake (4)Swine (301)Turkey (57)Whale (3)Yeast (5336)Zebrafish (3022)Isotype (156643)
IgA (13624)IgA1 (941)IgA2 (318)IgD (1949)IgE (5594)IgG (87187)IgG1 (16733)IgG2 (1329)IgG3 (2719)IgG4 (1689)IgM (22029)IgY (2531)Label (239340)
AF488 (2465)AF594 (662)AF647 (2324)ALEXA (11546)ALEXA FLUOR 350 (255)ALEXA FLUOR 405 (260)ALEXA FLUOR 488 (672)ALEXA FLUOR 532 (260)ALEXA FLUOR 555 (274)ALEXA FLUOR 568 (253)ALEXA FLUOR 594 (299)ALEXA FLUOR 633 (262)ALEXA FLUOR 647 (607)ALEXA FLUOR 660 (252)ALEXA FLUOR 680 (422)ALEXA FLUOR 700 (2)ALEXA FLUOR 750 (414)ALEXA FLUOR 790 (215)Alkaline Phosphatase (825)Allophycocyanin (32)ALP (387)AMCA (80)AP (1160)APC (15217)APC C750 (13)Apc Cy7 (1248)ATTO 390 (3)ATTO 488 (6)ATTO 550 (1)ATTO 594 (5)ATTO 647N (4)AVI (53)Beads (225)Beta Gal (2)BgG (1)BIMA (6)Biotin (27817)Biotinylated (1810)Blue (708)BSA (878)BTG (46)C Terminal (688)CF Blue (19)Colloidal (22)Conjugated (29246)Cy (163)Cy3 (390)Cy5 (2041)Cy5 5 (2469)Cy5 PE (1)Cy7 (3638)Dual (170)DY549 (3)DY649 (3)Dye (1)DyLight (1430)DyLight 405 (7)DyLight 488 (216)DyLight 549 (17)DyLight 594 (84)DyLight 649 (3)DyLight 650 (35)DyLight 680 (17)DyLight 800 (21)Fam (5)Fc Tag (8)FITC (30165)Flag (208)Fluorescent (146)GFP (563)GFP Tag (164)Glucose Oxidase (59)Gold (511)Green (580)GST (711)GST Tag (315)HA Tag (430)His (619)His Tag (492)Horseradish (550)HRP (12960)HSA (249)iFluor (16571)Isoform b (31)KLH (88)Luciferase (105)Magnetic (254)MBP (338)MBP Tag (87)Myc Tag (398)OC 515 (1)Orange (78)OVA (104)Pacific Blue (213)Particle (64)PE (33571)PerCP (8438)Peroxidase (1380)POD (11)Poly Hrp (92)Poly Hrp40 (13)Poly Hrp80 (3)Puro (32)Red (2440)RFP Tag (63)Rhodamine (607)RPE (910)S Tag (194)SCF (184)SPRD (351)Streptavidin (55)SureLight (77)T7 Tag (97)Tag (4710)Texas (1249)Texas Red (1231)Triple (10)TRITC (1401)TRX tag (87)Unconjugated (2110)Unlabeled (218)Yellow (84)Pathogen (489613)
Adenovirus (8665)AIV (315)Bordetella (25035)Borrelia (18281)Candida (17817)Chikungunya (638)Chlamydia (17650)CMV (121394)Coronavirus (5948)Coxsackie (854)Dengue (2868)EBV (1510)Echovirus (215)Enterovirus (677)Hantavirus (254)HAV (905)HBV (2095)HHV (873)HIV (7865)hMPV (300)HSV (2356)HTLV (634)Influenza (22132)Isolate (1208)KSHV (396)Lentivirus (3755)Lineage (3025)Lysate (127759)Marek (93)Measles (1163)Parainfluenza (1681)Poliovirus (3030)Poxvirus (74)Rabies (1519)Reovirus (527)Retrovirus (1069)Rhinovirus (507)Rotavirus (5346)RSV (1781)Rubella (1070)SIV (277)Strain (67790)Vaccinia (7233)VZV (666)WNV (363)Species (2982223)
Alligator (10)Bovine (159546)Canine (120648)Cat (13082)Chicken (113771)Cod (1)Cow (2030)Dog (12745)Dolphin (21)Duck (9567)Equine (2004)Feline (996)Ferret (259)Fish (12797)Frog (1)Goat (90451)Guinea Pig (87888)Hamster (36959)Horse (41226)Human (955186)Insect (653)Lemur (119)Lizard (24)Monkey (110914)Mouse (470743)Pig (26204)Porcine (131703)Rabbit (127597)Rat (347841)Ray (442)Salmon (348)Seal (8)Shark (29)Sheep (104984)Snake (12)Swine (511)Toad (4)Turkey (244)Turtle (75)Whale (45)Zebrafish (535)Technique (5597646)
Activation (170393)Activity (10733)Affinity (44631)Agarose (2604)Aggregation (199)Antigen (135358)Apoptosis (27447)Array (2022)Blocking (71767)Blood (8528)Blot (10966)ChiP (815)Chromatin (6286)Colorimetric (9913)Control (80065)Culture (3218)Cytometry (5481)Depletion (54)DNA (172449)Dot (233)EIA (1039)Electron (6275)Electrophoresis (254)Elispot (1294)Enzymes (52671)Exosome (4280)Extract (1090)Fab (2230)FACS (43)FC (80929)Flow (6666)Fluorometric (1407)Formalin (97)Frozen (2671)Functional (708)Gel (2484)HTS (136)IF (12906)IHC (16566)Immunoassay (1589)Immunofluorescence (4119)Immunohistochemistry (72)Immunoprecipitation (68)intracellular (5602)IP (2840)iPSC (259)Isotype (8791)Lateral (1585)Lenti (319416)Light (37250)Microarray (47)MicroRNA (4834)Microscopy (52)miRNA (88044)Monoclonal (516109)Multi (3844)Multiplex (302)Negative (4261)PAGE (2520)Panel (1520)Paraffin (2587)PBS (20270)PCR (9)Peptide (276160)PerCP (13759)Polyclonal (2762994)Positive (6335)Precipitation (61)Premix (130)Primers (3467)Probe (2627)Profile (229)Pure (7808)Purification (15)Purified (78305)Real Time (3042)Resin (2955)Reverse (2435)RIA (460)RNAi (17)Rox (1022)RT PCR (6608)Sample (2667)SDS (1527)Section (2895)Separation (86)Sequencing (122)Shift (22)siRNA (319447)Standard (42468)Sterile (10170)Strip (1863)Taq (2)Tip (1176)Tissue (42812)Tube (3306)Vitro (3577)Vivo (981)WB (2515)Western Blot (10683)Tissue (2015946)
Adenocarcinoma (1075)Adipose (3459)Adrenal (657)Adult (4883)Amniotic (65)Animal (2447)Aorta (436)Appendix (89)Array (2022)Ascites (4377)Bile Duct (20)Bladder (1672)Blood (8528)Bone (27330)Brain (31189)Breast (10917)Calvaria (28)Carcinoma (13493)cDNA (58547)Cell (413805)Cellular (9357)Cerebellum (700)Cervix (232)Child (1)Choroid (19)Colon (3911)Connective (3601)Contaminant (3)Control (80065)Cord (661)Corpus (148)Cortex (698)Dendritic (1849)Diseased (265)Donor (1360)Duct (861)Duodenum (643)Embryo (425)Embryonic (4583)Endometrium (463)Endothelium (1424)Epidermis (166)Epithelium (4221)Esophagus (716)Exosome (4280)Eye (2033)Female (475)Frozen (2671)Gallbladder (155)Genital (5)Gland (3436)Granulocyte (8981)Heart (6850)Hela (413)Hippocampus (325)Histiocytic (74)Ileum (201)Insect (4880)Intestine (1944)Isolate (1208)Jejunum (175)Kidney (8075)Langerhans (283)Leukemia (21541)Liver (17340)Lobe (835)Lung (6064)Lymph (1208)Lymphatic (639)lymphocyte (22572)Lymphoma (12782)Lysate (127759)Lysosome (2813)Macrophage (31794)Male (1617)Malignant (1465)Mammary (1985)Mantle (1042)Marrow (2210)Mastocytoma (3)Matched (11710)Medulla (156)Melanoma (15522)Membrane (105772)Metastatic (3574)Mitochondrial (160319)Muscle (37419)Myeloma (748)Myocardium (11)Nerve (6398)Neuronal (17028)Node (1206)Normal (9486)Omentum (10)Ovarian (2509)Ovary (1172)Pair (47185)Pancreas (2843)Panel (1520)Penis (64)Peripheral (1912)Pharynx (122)Pituitary (5411)Placenta (4038)Prostate (9423)Proximal (318)Rectum (316)Region (202210)Retina (956)Salivary (3119)Sarcoma (6946)Section (2895)Serum (24880)Set (167654)Skeletal (13628)Skin (1879)Smooth (7577)Spinal (424)Spleen (2292)Stem (8892)Stomach (925)Stroma (49)Subcutaneous (47)Testis (15393)Thalamus (127)Thoracic (60)Throat (40)Thymus (2986)Thyroid (14121)Tongue (140)Total (10135)Trachea (227)Transformed (175)Tubule (48)Tumor (76921)Umbilical (208)Ureter (73)Urinary (2466)Uterine (303)Uterus (414)Unlocking Your Genetic Blueprint: The Genomic Revolution in Personalized Healthcare
Discover how cutting-edge genomic technologies are transforming medicine from a "one-size-fits-all" approach to truly individualized care.
Genprice
Scientific Publications

Unlocking Your Genetic Blueprint: The Genomic Revolution in Personalized Healthcare
Key Insights into Personalized Medicine's Genomic Revolution
Next-Generation Sequencing (NGS) as the Core Driver: NGS technology is the engine behind personalized medicine, enabling rapid, accurate, and cost-effective sequencing of vast amounts of genetic data, making comprehensive genomic profiling a reality.
Tailoring Treatments for Unprecedented Efficacy: Genomic data empowers healthcare providers to move beyond generalized treatments, allowing for precise diagnoses, targeted therapies, and proactive prevention strategies specifically designed for an individual's unique genetic makeup.
Integration with AI for Advanced Insights: The synergy between genomic data and artificial intelligence (AI) is crucial for interpreting complex datasets, uncovering subtle patterns, and developing predictive models that further enhance diagnostic accuracy and therapeutic effectiveness.
The landscape of healthcare is undergoing a profound transformation, shifting from a generalized approach to one that is deeply personal and precise. At the forefront of this revolution is the integration of genomic data, particularly through the remarkable advancements of Next-Generation Sequencing (NGS). This paradigm shift, often referred to as personalized or precision medicine, promises to redefine how diseases are prevented, diagnosed, and treated, moving us closer to truly tailored healthcare solutions.
The Dawn of Personalized Medicine: A Paradigm Shift
Personalized medicine marks a significant departure from conventional healthcare models. Instead of applying a uniform treatment strategy to all patients with a given condition, it leverages an individual's unique genetic profile, alongside other critical data such as lifestyle and environmental factors, to guide medical decisions. This bespoke approach aims to maximize therapeutic effectiveness, minimize adverse reactions, and ultimately improve patient outcomes by aligning interventions with a person's biological individuality.
The central tenet of personalized medicine is the recognition that each individual responds differently to treatments and possesses unique susceptibilities to diseases. Genomic data provides the crucial insights needed to understand these individual differences, offering a window into the molecular underpinnings of health and disease.
Beyond "One-Size-Fits-All": The Precision Promise
The promise of precision medicine lies in its ability to offer highly specific interventions. For instance, in oncology, understanding the genetic mutations driving a patient's tumor can lead to the selection of targeted therapies that specifically attack those mutated cells, leaving healthy cells largely unharmed. This contrasts sharply with traditional chemotherapy, which often has broad, systemic effects.
Next-Generation Sequencing: The Engine of Genomic Insight
The technological backbone of personalized medicine is Next-Generation Sequencing (NGS). NGS represents a monumental leap forward from older sequencing methods, such as Sanger sequencing. It allows for the simultaneous sequencing of millions of DNA fragments, generating vast quantities of genetic data with unparalleled speed, accuracy, and cost-effectiveness. This capability has made comprehensive genetic profiling accessible, enabling a deeper understanding of complex diseases.
How NGS Works: A Glimpse into the Process
NGS technologies fundamentally differ from their predecessors by enabling massively parallel sequencing. Instead of sequencing DNA fragments one by one, NGS platforms can process thousands to millions of reactions concurrently. This high-throughput capability is crucial for analyzing entire genomes, exomes (the protein-coding regions of the genome), or specific gene panels, providing a holistic view of an individual's genetic landscape.

Key Advantages of NGS:
- Speed: NGS can sequence entire genomes in a fraction of the time compared to older methods.
- Cost-Effectiveness: While initially expensive, the cost of NGS has plummeted, making it increasingly viable for routine clinical use.
- High-Throughput: It can process a massive amount of genetic material simultaneously, from small panels to whole genomes.
- Accuracy and Sensitivity: NGS offers high-resolution data, capable of detecting rare variants and low-frequency mutations, which are critical for certain diseases.

This radar chart illustrates the comparative impact of Next-Generation Sequencing (NGS) versus traditional sequencing methods across several key dimensions relevant to personalized medicine. NGS excels in speed, cost-effectiveness (relative to the volume of data generated), diagnostic accuracy, and its potential to guide targeted treatments and disease prediction.
Translating Genomic Data into Targeted Care
The true power of genomic data emerges when it is translated into actionable clinical insights. This translation forms the core of personalized medicine, moving beyond the general to the individual.
Applications Across Medical Fields:
-Oncology: In cancer treatment, NGS is invaluable for identifying specific genetic mutations within tumor cells. This allows oncologists to select targeted therapies, immunotherapies, or even recommend participation in clinical trials that are precisely matched to the tumor's unique genetic profile. This approach has led to significant improvements in response rates and reduced toxicity compared to conventional chemotherapy.
-Pharmacogenomics: This field uses an individual's genetic information to predict their response to specific medications and their likelihood of experiencing adverse drug reactions. By understanding how a patient metabolizes certain drugs, healthcare providers can optimize drug dosages or select alternative medications, thereby enhancing efficacy and safety.
-Rare Genetic Diseases: For patients suffering from rare and undiagnosed genetic disorders, NGS can rapidly identify the causative genetic variants, often ending years of diagnostic uncertainty and enabling the development of personalized management plans.
-Cardiovascular and Neurodegenerative Diseases: Genomic insights are increasingly being used to assess an individual's risk for complex conditions like heart disease or Alzheimer's. This allows for proactive prevention strategies, early monitoring, and tailored interventions to mitigate disease progression.
-Infectious Diseases: NGS can rapidly identify pathogens and their resistance profiles, guiding the selection of the most effective antimicrobial treatments.
The Synergy of Genomics and Artificial Intelligence
The vast datasets generated by NGS, often referred to as "big data," present both immense opportunities and significant challenges. Interpreting this complex information effectively requires sophisticated analytical tools. This is where artificial intelligence (AI) and machine learning come into play, forming a powerful synergy with genomics.

ScienceDirecte: Artificial intelligence and machine learning in precision medicine: A paradigm shift in big data analysis
Unlocking Deeper Insights with AI:
AI algorithms can sift through massive genomic datasets, identifying intricate patterns, subtle biomarkers, and complex correlations that might be missed by human analysis. This capability is crucial for:
-Biomarker Discovery: AI can help pinpoint novel genetic biomarkers that indicate disease risk, progression, or response to therapy.
-Predictive Modeling: Machine learning models can predict disease susceptibility, drug efficacy, and potential adverse effects based on an individual's genomic profile.
-Dr ug Discovery and Development: AI can accelerate the identification of new drug targets and optimize drug design by analyzing genetic pathways associated with diseases.
-Real-Time Data Analysis: The integration of AI allows for quicker interpretation of genomic results, facilitating more timely and effective clinical decisions.
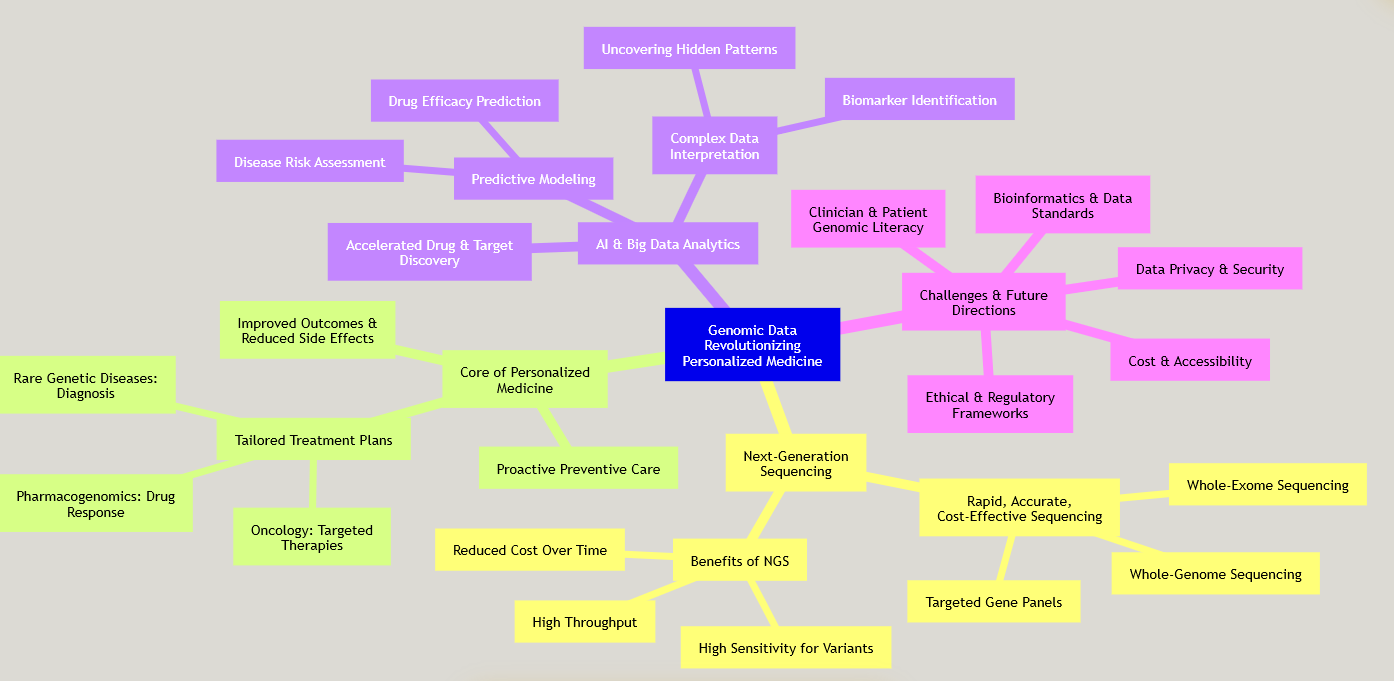
This mindmap illustrates the interconnected components of how genomic data, primarily driven by Next-Generation Sequencing (NGS), is revolutionizing personalized medicine, highlighting its core applications, the role of AI, and the ongoing challenges.
Challenges and Future Directions
Despite the immense promise, the widespread adoption of genomic medicine faces several hurdles that require concerted effort from researchers, clinicians, policymakers, and industry stakeholders.
Key Challenges:
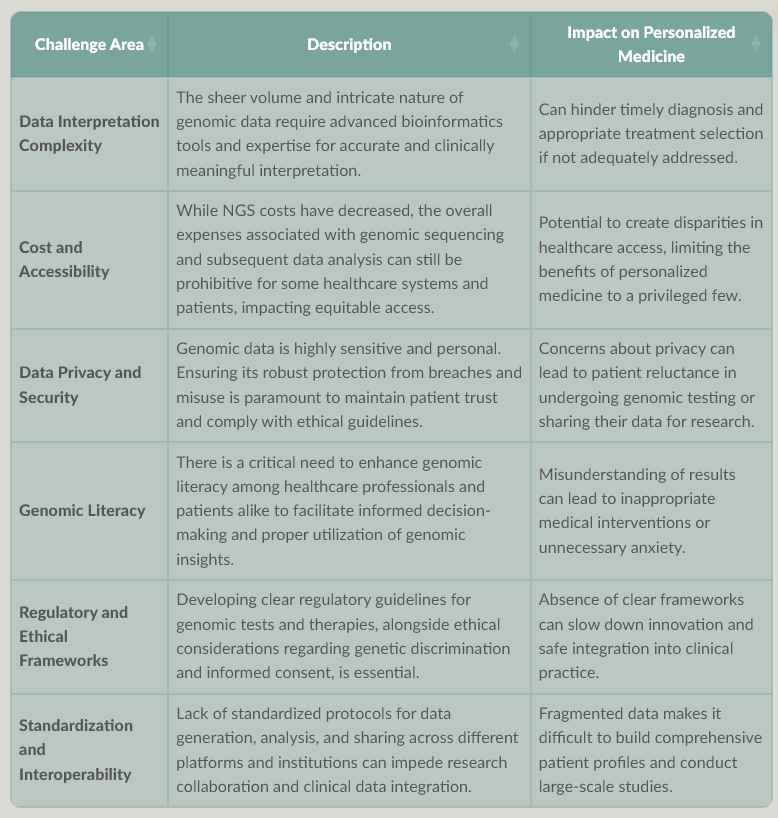
This table summarizes the significant challenges that must be overcome for the widespread and equitable implementation of personalized medicine, driven by genomic data.
The Path Forward:
The future of personalized medicine is undoubtedly collaborative and technologically empowered. Ongoing efforts are focused on:
-Robust Evidence Generation: Conducting rigorous clinical trials to build a strong evidence base for the efficacy and cost-effectiveness of genomic-guided interventions.
-Bioinformatics Advancements: Developing more intuitive and powerful bioinformatics tools to streamline data analysis and interpretation.
-Policy and Reimbursement: Establishing policies that support the integration of genomic testing into routine care and ensuring adequate reimbursement mechanisms.
-Education and Training: Investing in education for both clinicians and the public to foster a deeper understanding of genomic medicine.

This bar chart visually represents the perceived severity of various challenges currently facing the widespread implementation of personalized medicine, based on an opinionated analysis. Data privacy concerns and the complexity of data interpretation are seen as particularly significant hurdles.
Looking Ahead: The Future of Health is Personal
The trajectory of genomic medicine is clear: it is moving towards more precise, proactive, and participatory healthcare. As sequencing technologies continue to advance and become more affordable, genomic data will increasingly inform preventive strategies, facilitate earlier diagnoses, and guide personalized treatment pathways across a spectrum of diseases. The integration of genomic insights into everyday clinical practice is not a distant dream but an evolving reality.
The global personalized medicine market is projected to experience substantial growth, reflecting the increasing recognition of its value. This expansion is fueled by continuous innovations in genomics, the burgeoning role of AI, and a deeper understanding of human biology.
This video, "The Future of Precision Medicine: Stem Cells, Gene Therapy...", provides an overview of key advancements and future trends in precision medicine, including the role of AI and gene therapy, which are integral to leveraging genomic data effectively.
Frequently Asked Questions
-What is personalized medicine?
Personalized medicine, also known as precision medicine, is a medical model that tailors healthcare decisions, treatments, and prevention strategies to each individual based on their unique genetic makeup, lifestyle, and environment. It moves away from a "one-size-fits-all" approach.
-How does Next-Generation Sequencing (NGS) contribute to personalized medicine?
NGS is a technology that allows for the rapid, accurate, and cost-effective sequencing of large amounts of DNA or RNA. In personalized medicine, it identifies specific genetic variations, mutations, and biomarkers that influence disease risk, progression, and response to treatment, enabling highly targeted interventions.
-What are the main applications of genomic data in healthcare?
Genomic data is applied across various fields, including oncology (for targeted cancer therapies), pharmacogenomics (to predict drug response), diagnosis of rare genetic diseases, risk assessment for cardiovascular and neurodegenerative conditions, and guiding treatment for infectious diseases.
-What role does AI play in genomic medicine?
AI and machine learning are critical for interpreting the vast and complex datasets generated by NGS. They help in identifying patterns, discovering biomarkers, building predictive models for disease risk and drug efficacy, and accelerating drug discovery and development.
-What are the primary challenges in implementing personalized medicine broadly?
Key challenges include the complexity of genomic data interpretation, the cost and accessibility of testing, ensuring data privacy and security, the need for enhanced genomic literacy among clinicians and patients, and the development of robust regulatory and ethical frameworks.
Conclusion
The revolution spearheaded by genomic data and Next-Generation Sequencing is undeniably reshaping personalized medicine. By providing an unprecedented depth of insight into an individual's genetic blueprint, these technologies are enabling a new era of healthcare characterized by precision, efficacy, and personalization. While challenges remain in data interpretation, accessibility, and ethical considerations, the continuous advancements in sequencing, bioinformatics, and artificial intelligence promise a future where medical interventions are not just effective, but perfectly tailored to each patient.
References
-frontiersin.org: Advancing Personalized Medicine Through the Application of Whole Exome Sequencing and Big Data Analytics - Frontiers
-bmcmedgenomics.biomedcentral.com: From big data analysis to personalized medicine for all: challenges and opportunities - BMC Medical Genomics
-genome.gov: Genomics and Medicine (Genome.gov) - Genome.gov
-sciencedirect.com: Next-Generation sequencing transforming clinical practice and precision medicine
-nature.com: Pharmacogenomics and Personalized Medicine | Learn Science at Scitable
-en.wikipedia.org: Personalized genomics
Tags
- Next-Generation Sequencing
- NGS
- One-Size-Fits-All
- Genomic medicine
- Personalized medicine
- Personalized genomics
