Filters
Host (768597)
Bovine (1090)Canine (20)Cat (408)Chicken (1642)Cod (2)Cow (333)Crab (15)Dog (524)Dolphin (2)Duck (13)E Coli (239129)Equine (7)Feline (1864)Ferret (306)Fish (125)Frog (55)Goat (36847)Guinea Pig (752)Hamster (1376)Horse (903)Insect (2053)Mammalian (512)Mice (6)Monkey (601)Mouse (96266)Pig (197)Porcine (70)Rabbit (358709)Rat (11723)Ray (55)Salamander (4)Salmon (15)Shark (3)Sheep (4247)Snake (4)Swine (301)Turkey (57)Whale (3)Yeast (5336)Zebrafish (3022)Isotype (156643)
IgA (13624)IgA1 (941)IgA2 (318)IgD (1949)IgE (5594)IgG (87187)IgG1 (16733)IgG2 (1329)IgG3 (2719)IgG4 (1689)IgM (22029)IgY (2531)Label (239340)
AF488 (2465)AF594 (662)AF647 (2324)ALEXA (11546)ALEXA FLUOR 350 (255)ALEXA FLUOR 405 (260)ALEXA FLUOR 488 (672)ALEXA FLUOR 532 (260)ALEXA FLUOR 555 (274)ALEXA FLUOR 568 (253)ALEXA FLUOR 594 (299)ALEXA FLUOR 633 (262)ALEXA FLUOR 647 (607)ALEXA FLUOR 660 (252)ALEXA FLUOR 680 (422)ALEXA FLUOR 700 (2)ALEXA FLUOR 750 (414)ALEXA FLUOR 790 (215)Alkaline Phosphatase (825)Allophycocyanin (32)ALP (387)AMCA (80)AP (1160)APC (15217)APC C750 (13)Apc Cy7 (1248)ATTO 390 (3)ATTO 488 (6)ATTO 550 (1)ATTO 594 (5)ATTO 647N (4)AVI (53)Beads (225)Beta Gal (2)BgG (1)BIMA (6)Biotin (27817)Biotinylated (1810)Blue (708)BSA (878)BTG (46)C Terminal (688)CF Blue (19)Colloidal (22)Conjugated (29246)Cy (163)Cy3 (390)Cy5 (2041)Cy5 5 (2469)Cy5 PE (1)Cy7 (3638)Dual (170)DY549 (3)DY649 (3)Dye (1)DyLight (1430)DyLight 405 (7)DyLight 488 (216)DyLight 549 (17)DyLight 594 (84)DyLight 649 (3)DyLight 650 (35)DyLight 680 (17)DyLight 800 (21)Fam (5)Fc Tag (8)FITC (30165)Flag (208)Fluorescent (146)GFP (563)GFP Tag (164)Glucose Oxidase (59)Gold (511)Green (580)GST (711)GST Tag (315)HA Tag (430)His (619)His Tag (492)Horseradish (550)HRP (12960)HSA (249)iFluor (16571)Isoform b (31)KLH (88)Luciferase (105)Magnetic (254)MBP (338)MBP Tag (87)Myc Tag (398)OC 515 (1)Orange (78)OVA (104)Pacific Blue (213)Particle (64)PE (33571)PerCP (8438)Peroxidase (1380)POD (11)Poly Hrp (92)Poly Hrp40 (13)Poly Hrp80 (3)Puro (32)Red (2440)RFP Tag (63)Rhodamine (607)RPE (910)S Tag (194)SCF (184)SPRD (351)Streptavidin (55)SureLight (77)T7 Tag (97)Tag (4710)Texas (1249)Texas Red (1231)Triple (10)TRITC (1401)TRX tag (87)Unconjugated (2110)Unlabeled (218)Yellow (84)Pathogen (489613)
Adenovirus (8665)AIV (315)Bordetella (25035)Borrelia (18281)Candida (17817)Chikungunya (638)Chlamydia (17650)CMV (121394)Coronavirus (5948)Coxsackie (854)Dengue (2868)EBV (1510)Echovirus (215)Enterovirus (677)Hantavirus (254)HAV (905)HBV (2095)HHV (873)HIV (7865)hMPV (300)HSV (2356)HTLV (634)Influenza (22132)Isolate (1208)KSHV (396)Lentivirus (3755)Lineage (3025)Lysate (127759)Marek (93)Measles (1163)Parainfluenza (1681)Poliovirus (3030)Poxvirus (74)Rabies (1519)Reovirus (527)Retrovirus (1069)Rhinovirus (507)Rotavirus (5346)RSV (1781)Rubella (1070)SIV (277)Strain (67790)Vaccinia (7233)VZV (666)WNV (363)Species (2982223)
Alligator (10)Bovine (159546)Canine (120648)Cat (13082)Chicken (113771)Cod (1)Cow (2030)Dog (12745)Dolphin (21)Duck (9567)Equine (2004)Feline (996)Ferret (259)Fish (12797)Frog (1)Goat (90451)Guinea Pig (87888)Hamster (36959)Horse (41226)Human (955186)Insect (653)Lemur (119)Lizard (24)Monkey (110914)Mouse (470743)Pig (26204)Porcine (131703)Rabbit (127597)Rat (347841)Ray (442)Salmon (348)Seal (8)Shark (29)Sheep (104984)Snake (12)Swine (511)Toad (4)Turkey (244)Turtle (75)Whale (45)Zebrafish (535)Technique (5597646)
Activation (170393)Activity (10733)Affinity (44631)Agarose (2604)Aggregation (199)Antigen (135358)Apoptosis (27447)Array (2022)Blocking (71767)Blood (8528)Blot (10966)ChiP (815)Chromatin (6286)Colorimetric (9913)Control (80065)Culture (3218)Cytometry (5481)Depletion (54)DNA (172449)Dot (233)EIA (1039)Electron (6275)Electrophoresis (254)Elispot (1294)Enzymes (52671)Exosome (4280)Extract (1090)Fab (2230)FACS (43)FC (80929)Flow (6666)Fluorometric (1407)Formalin (97)Frozen (2671)Functional (708)Gel (2484)HTS (136)IF (12906)IHC (16566)Immunoassay (1589)Immunofluorescence (4119)Immunohistochemistry (72)Immunoprecipitation (68)intracellular (5602)IP (2840)iPSC (259)Isotype (8791)Lateral (1585)Lenti (319416)Light (37250)Microarray (47)MicroRNA (4834)Microscopy (52)miRNA (88044)Monoclonal (516109)Multi (3844)Multiplex (302)Negative (4261)PAGE (2520)Panel (1520)Paraffin (2587)PBS (20270)PCR (9)Peptide (276160)PerCP (13759)Polyclonal (2762994)Positive (6335)Precipitation (61)Premix (130)Primers (3467)Probe (2627)Profile (229)Pure (7808)Purification (15)Purified (78305)Real Time (3042)Resin (2955)Reverse (2435)RIA (460)RNAi (17)Rox (1022)RT PCR (6608)Sample (2667)SDS (1527)Section (2895)Separation (86)Sequencing (122)Shift (22)siRNA (319447)Standard (42468)Sterile (10170)Strip (1863)Taq (2)Tip (1176)Tissue (42812)Tube (3306)Vitro (3577)Vivo (981)WB (2515)Western Blot (10683)Tissue (2015946)
Adenocarcinoma (1075)Adipose (3459)Adrenal (657)Adult (4883)Amniotic (65)Animal (2447)Aorta (436)Appendix (89)Array (2022)Ascites (4377)Bile Duct (20)Bladder (1672)Blood (8528)Bone (27330)Brain (31189)Breast (10917)Calvaria (28)Carcinoma (13493)cDNA (58547)Cell (413805)Cellular (9357)Cerebellum (700)Cervix (232)Child (1)Choroid (19)Colon (3911)Connective (3601)Contaminant (3)Control (80065)Cord (661)Corpus (148)Cortex (698)Dendritic (1849)Diseased (265)Donor (1360)Duct (861)Duodenum (643)Embryo (425)Embryonic (4583)Endometrium (463)Endothelium (1424)Epidermis (166)Epithelium (4221)Esophagus (716)Exosome (4280)Eye (2033)Female (475)Frozen (2671)Gallbladder (155)Genital (5)Gland (3436)Granulocyte (8981)Heart (6850)Hela (413)Hippocampus (325)Histiocytic (74)Ileum (201)Insect (4880)Intestine (1944)Isolate (1208)Jejunum (175)Kidney (8075)Langerhans (283)Leukemia (21541)Liver (17340)Lobe (835)Lung (6064)Lymph (1208)Lymphatic (639)lymphocyte (22572)Lymphoma (12782)Lysate (127759)Lysosome (2813)Macrophage (31794)Male (1617)Malignant (1465)Mammary (1985)Mantle (1042)Marrow (2210)Mastocytoma (3)Matched (11710)Medulla (156)Melanoma (15522)Membrane (105772)Metastatic (3574)Mitochondrial (160319)Muscle (37419)Myeloma (748)Myocardium (11)Nerve (6398)Neuronal (17028)Node (1206)Normal (9486)Omentum (10)Ovarian (2509)Ovary (1172)Pair (47185)Pancreas (2843)Panel (1520)Penis (64)Peripheral (1912)Pharynx (122)Pituitary (5411)Placenta (4038)Prostate (9423)Proximal (318)Rectum (316)Region (202210)Retina (956)Salivary (3119)Sarcoma (6946)Section (2895)Serum (24880)Set (167654)Skeletal (13628)Skin (1879)Smooth (7577)Spinal (424)Spleen (2292)Stem (8892)Stomach (925)Stroma (49)Subcutaneous (47)Testis (15393)Thalamus (127)Thoracic (60)Throat (40)Thymus (2986)Thyroid (14121)Tongue (140)Total (10135)Trachea (227)Transformed (175)Tubule (48)Tumor (76921)Umbilical (208)Ureter (73)Urinary (2466)Uterine (303)Uterus (414)Molecular Dissection of Aggresophagy: Identification of a Central Enzyme Regulating Aggresome Clearance
Cells need ways to deal with damaged or misfolded proteins. When these proteins build up, they can clump together into structures called aggresomes. If not removed, these clumps can harm the cell. A special process called autophagy helps get rid of them. Scientists have now found a key enzyme that helps cells start this process—an important step in understanding how cells clean up harmful waste.
Genprice
Scientific Publications
.jpg)
Molecular Dissection of Aggresophagy: Identification of a Central Enzyme Regulating Aggresome Clearance
What Are Aggresomes?
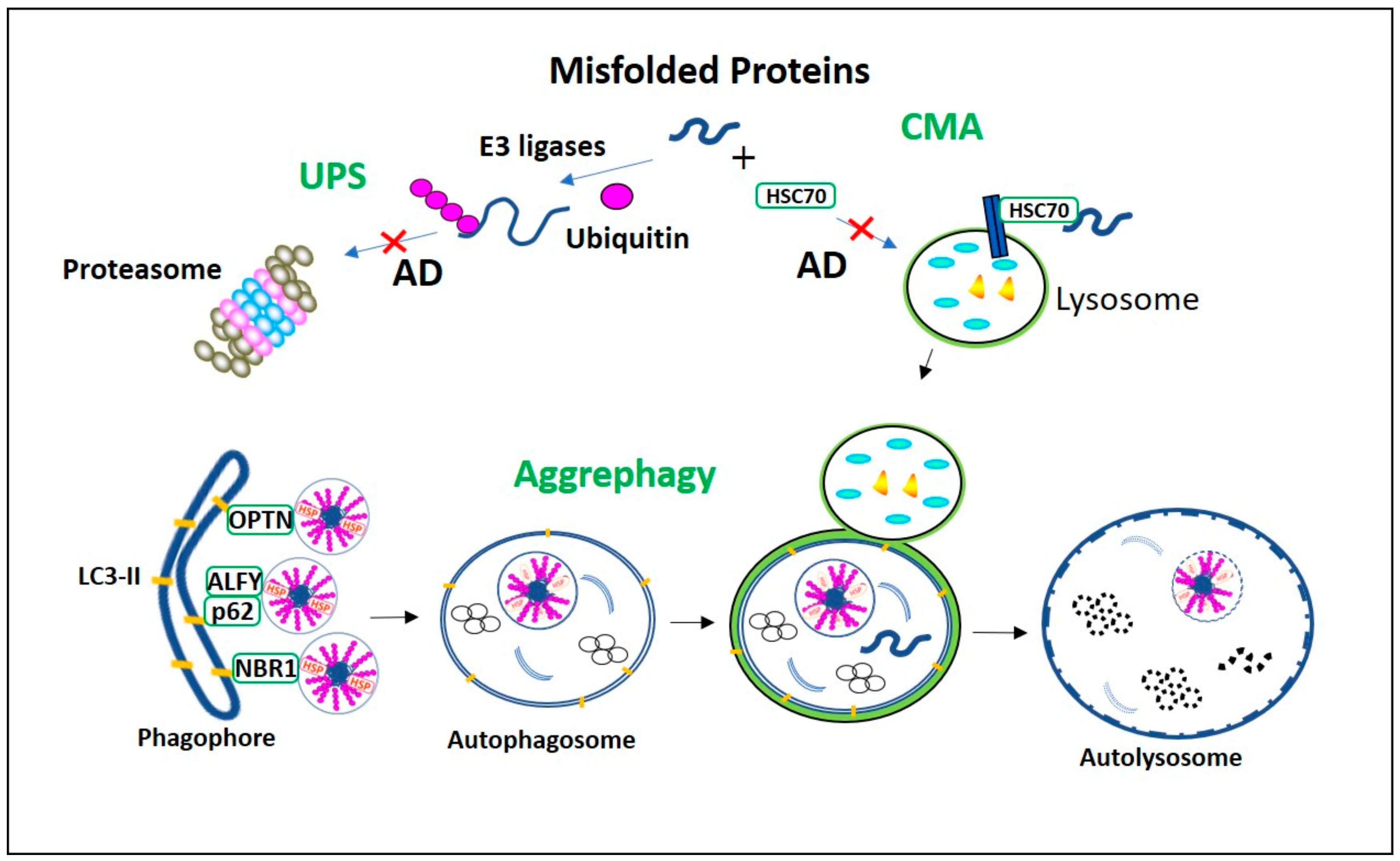
When the cell’s first line of defense—the ubiquitin-proteasome system (UPS) is unable to break down damaged or misfolded proteins, the cell needs a backup plan. In such cases, misfolded proteins are collected into a centralized location in the cell called the microtubule-organizing center (MTOC). These collections are called aggresomes.
Forming aggresomes helps protect the rest of the cell from the toxic effects of protein aggregates. However, aggresomes are not a permanent solution they must be cleared to prevent long-term damage.
To clear them, the cell uses a process called aggrephagy a form of autophagy that specifically targets aggresomes for degradation.
The Role of Autophagy and Aggrephagy
Autophagy is a process cells use to break down and recycle components, including damaged organelles, protein aggregates, and even invading bacteria. During autophagy, cellular material is enclosed within a membrane, forming a structure called an autophagosome, which then fuses with a lysosome where the contents are broken down and recycled.
Aggrephagy is a type of selective autophagy. It specifically targets protein aggregates, including aggresomes. For this to happen, several things must occur:
- The cell must identify which proteins or aggregates need to be removed.
- The aggresomes must be moved to the right location.
- Specialized receptors must recruit autophagy machinery to form autophagosomes.
- These autophagosomes must fuse with lysosomes to allow for degradation.
Until recently, scientists didn’t fully understand what triggered this entire process or which molecules were essential for getting it started. That’s where this new discovery comes in.
A Key Enzyme Identified
Researchers have now identified a specific enzyme that plays a central role in aggresophagy. While the exact name of the enzyme can vary depending on the study (for example, some studies focus on HDAC6, USP10, or TRIM50), the general finding is that this enzyme performs several essential tasks:
- It recognizes ubiquitinated proteins, meaning proteins that have been tagged for removal.
- It helps transport aggresomes to the perinuclear region of the cell.
- It helps recruit autophagy receptor proteins such as p62/SQSTM1, which are required to link the aggresome to the autophagic machinery.
- It works with proteins like LC3 to help form the autophagosome membrane around the aggresome.
If the enzyme is not working properly or if its levels are too low cells are much less efficient at clearing aggresomes. This can result in a buildup of toxic proteins, leading to cell stress, dysfunction, and even death.
Why Is This Important?
Protein aggregation and failure to clear these aggregates are seen in many neurodegenerative diseases, such as:
In these conditions, misfolded proteins form clumps in nerve cells and disrupt their function. If scientists can better understand the enzymes that help cells remove these clumps, they can look for ways to enhance or support this natural process. This could reduce cell stress and improve cell survival.
Beyond neurological conditions, improving aggresophagy might help in other diseases where cellular stress plays a role, including some types of cancer and inflammatory diseases.
What Comes Next?
Now that this enzyme has been identified, scientists are exploring several important questions:
- Can its activity be safely increased in cells that are under stress?
- How is this enzyme regulated under normal conditions and in disease?
- Are there natural molecules in the body that influence this enzyme’s function?
- Can supporting this pathway help slow down or prevent protein aggregation diseases?
Research is also continuing in model systems such as yeast, mice, and cultured human cells to understand how this enzyme works in different biological contexts.
